
Home » Transformative Science in Cancer » Immunotherapy in Cancer Treatment
Immunotherapy in Cancer Treatment
The immune system is a complex network of cells, organs, tissues, and proteins that defend the body against different infections. This system can recognize the body’s cells from foreign cells and this feature leads to an attack on the foreign cells like pathogens and cancer cells and their destruction.
Observation of cancer immunity is an important process in which the immune system identifies new tumor cells and destroys them. Commonly, the immune system identifies the tumor cells based on the tumor-related antigens (TAAs). There are different kinds of immune cells and pathways of a molecule that lead to the elimination of cancer cells. Cytotoxic T cells and Natural killer cells are a subgroup of white blood cells that have a key role in the battle with cancer. First, the immune system identifies and marks the cancer cells. After identifying the cancer cells, a type of T-cell called “T Helper Cell” signals the other cells in the immune system by releasing “Cytokine” and attracts them to the area of the tumor. Finally, Regulatory T-cells (Tregs) prevent the over-functioning of the immune system by suppressing it and clearing the body from external factors. However, tumor cells run away from the immune system using different mechanisms.
Contents
Mechanisms of Immune Escape in Cancer
Tumors can evade attacks from the immune system through various mechanisms.
1) Reduction of immunization with the reduction of surface antigen expression.
2) Measuring safety checkpoints on the surface of the cancer cell to suppress the activity of the immune cells.
3) Using immunosuppressive cells such as myeloid-derived suppressive cells (MDSCs) and Regulatory T-cells (Tregs) and also cytokines to create an immunosuppressive microenvironment.
4) Releasing acidic and toxic metabolites which control the activity of the immune cells in the tumor microenvironment
History of Immunotherapy
The first modern scientific progress in immunotherapy is often attributed to two German doctors called “Busch” and “Fehleisen” in the late 1800s. These doctors figured that the recovery process of cancer patients after being infected with certain kinds of bacteria, increases and after this experiment, these two researchers concluded that injecting these bacteria into cancer patients leads to the reduction in tumor size. Doctor William B. Coley, now known as the father of immunotherapy, tried to use the immune system to cure cancer for the first time in the late 1800s. He produced a bacterial cocktail including (Streptococcus and Serratia marcescens) known as the toxins of Coley which were for curing cancer patients, especially for children with sarcoma. Nowadays we all know that the effect of these toxins in destroying cancer is through the cytokine’s family including Interleukins, Interferons, and Chemokines. The next revolutionary wave in cancer immunotherapy came with a better understanding of the immune system surveillance process by which the innate immune cells, eliminate cancer cells. The recent discovery of Immune Checkpoint Inhibitors, like CTLA-4 and PD-1, brought the field of immunotherapy to the current era.
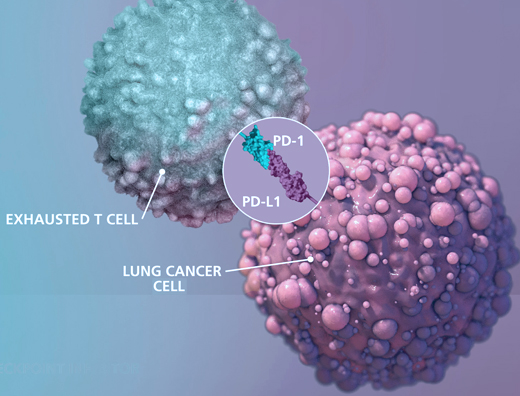
The Role of Immunotherapy in Cancer Treatment
Cancer is the second cause of human death after heart disease and the number of patients is still growing. Cancer treatment consists of the removal of tumor areas using surgery, radiotherapy, chemotherapy, and targeted therapy and in recent years immunotherapy has created some promising perspectives in cancer treatment. In immunotherapy, unlike traditional treatments such as chemotherapy and radiotherapy which directly target the disease, the body’s immune response helps the immune system fight cancer by boosting or changing how the immune system works to find and attack cancer cells. Immunotherapy-related treatments in different kinds of cancers (Blood cancers such as lymphoma, leukemia, multiple myeloma, and bladder, skin, kidney, and … cancers) have been highly successful. Some of these treatments might be effective for the most difficult and advanced cancers (such as lung cancer advanced pancreatic cancer and some kinds of colorectal cancer) and the effectivity level depends on different parameters such as the immune system of a patient, type, and stage of the cancer and treatment conditions.
In some cases, this treatment may be used in combination with other treatments such as surgery, chemotherapy, or radiotherapy for more effectiveness.
Types of Immunotherapy Methods in Cancer Treatment
There are different types of immunotherapy, each of which has its own approach and mechanism of action and consists of:
- Immune Checkpoint Inhibitors
- T-cell Therapy
- Cancer vaccines
- Cytokine Therapy
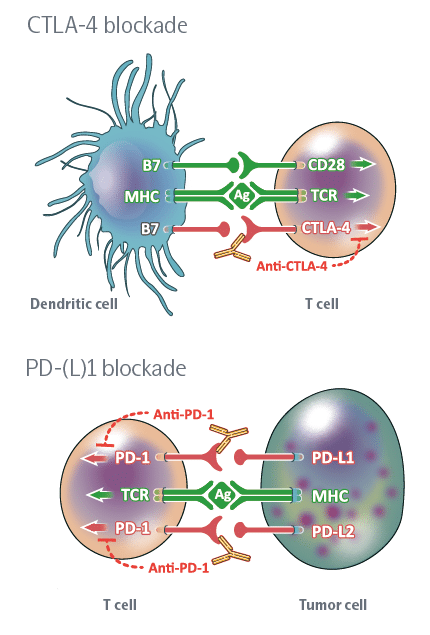
Immune Checkpoint Inhibitors
Cancer immunity is a dynamic process that describes the relationship between tumor cells and the immune system and is regulated by immune checkpoints, including immune surveillance and tumor progression.
Immune surveillance of tumors consists of three parts:
1) Tumor elimination
2) Balance between tumor and immune system
3) Immune system suppression
Immune checkpoints are proteins that act as an “off switch” of the immune system and prevent T-cells from attacking other cells in the body. Normally, certain checkpoints prevent the immune system from attacking healthy cells. Cancer cells can sometimes use these checkpoints to escape from the immune system, when immune checkpoints and cell surface proteins are connected, they send the “off” signal to the T-cells which leads to preventing the destruction of cancer by the immune system. For instance, PD_1 protein is a checkpoint protein on T-cells that leads to local immune system suppression after binding to its receptor on the surface of other cells. This receptor is highly expressed on the surface of cancer cells and this leads to the suppression of the immune system at the tumor area. Immune checkpoint inhibitors are a kind of immunotherapy that prevents the connection of immune checkpoint proteins to cancer cell surface proteins and lets the immune system identify cancer cells more effectively and attack them. This treatment method is used for cancers such as skin cancer, and melanoma and also as a treatment for NSCLC (kind of lung cancer). Durvalumab (Imfinzi), atezolizumab (Tecentriq), Nivolumab Pembrolizumab (Keytruda), Ipilimumab (Yervoy) and avelumab (Bavencio) can be mentioned among the drugs in this category.
Some of The Most Common Side Effects of Immune Checkpoint Inhibitors
Using this kind of drug is often associated with side effects related to the immune system, such as activation of the immune system and inflammatory response against the host’s healthy tissues. Some of the common side effects of this treatment include diarrhea, fatigue, cough, nausea, skin rashes, loss of appetite, constipation, and muscle and joint pain.
More Serious Side Effects That Rarely Occur Include:
Infusion reactions: Some people may have an infusion reaction while taking these drugs, which includes an allergic reaction and can include fever, chills, facial flushing, skin rash, itchy skin, dizziness, wheezing and shortness of breath.
Autoimmune reactions: These drugs practically destroy the regulatory function of the immune system and lead to the attack of the immune system on healthy tissues, for this reason, they increase the risk of developing autoimmune diseases such as type-1 diabetes.
T-Cell Therapy
T-cell Therapy is a new promising approach in terms of cancer treatment which includes the usage of the patient’s T-cell or engineered T-cells to target and destroy cancer cells. In this method, the patient’s T lymphocytes are used to fight cancer. In such a way that T cells are isolated from the patient’s blood and modified in the laboratory to produce specific receptors that allow them to recognize and attack cancer cells.
There are different kinds of treatments with T-cells, the two common methods are:
- Treatment with Chimeric antigen receptor cells (Car T-Cell Therapy)
- Treatment with Tumor-infiltrating lymphocytes (Tumor-infiltrating lymphocyte Therapy)
Car T-Cell Therapy
Treatment with chimeric antigen receptor cells is an immunotherapy that is used in the treatment of certain types of cancer, especially blood cancers such as leukemia and lymphoma. This treatment involves modifying the patient’s T cells to express a chimeric antigen receptor (CAR) on their surface. This receptor is designed to recognize specific antigens on the surface of cancer cells, enabling modified T cells to target and destroy cancer cells.
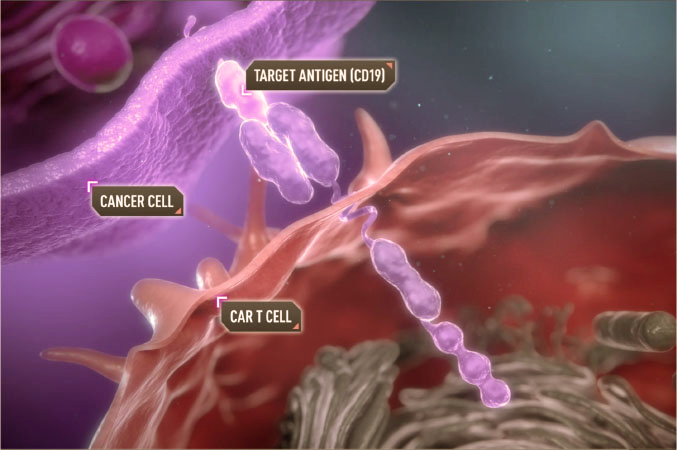
Examples of CAR-T Cell Therapy
Kymriah (Tisagenlecleucel): This medicine is one of the first CAR-T cell-based therapies approved by the FDA. It is used to treat children and young adult patients with B-cell Acute Lymphoblastic Leukemia (ALL) who have not responded to other treatments or whose disease has relapsed.
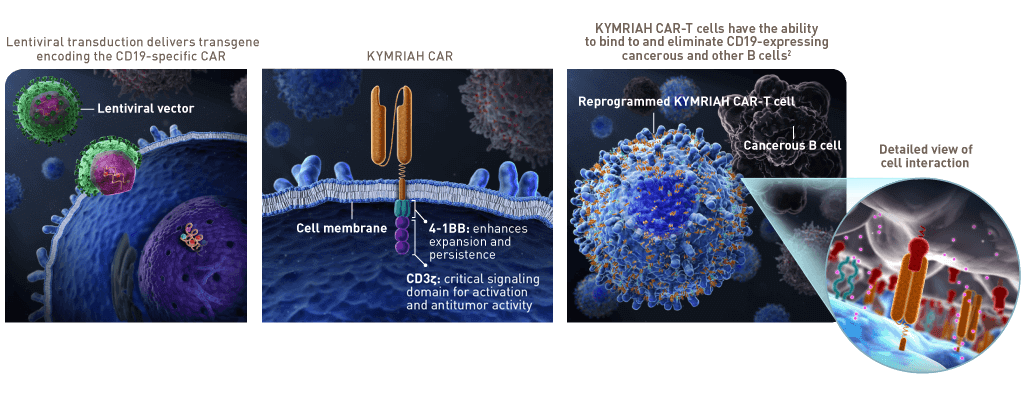
Yescarta (Axicabtagene Ciloleucel): This medicine is used to treat patients with relapsed or refractory large B-cell lymphoma and primary mediastinal large B-cell lymphoma.
Advantages of CAR-T Cell Therapy
Targeted therapy: CAR-T Cell Therapy offers a highly targeted approach to cancer treatment. CAR receptors are engineered to specifically recognize cancer cells, reduce damage to healthy cells, and minimize side effects associated with traditional therapies.
Long-term effects: CAR-T cells can remain in the body after treatment and continue to target cancer cells for a long time, potentially creating a long-lasting response in the body.
Disadvantages of CAR-T CELL THERAPY
Cytokine Release Syndrome (CRS): One of the major side effects of this procedure is cytokine release syndrome, a severe immune reaction that can lead to fever, flu-like symptoms, and even organ dysfunction. This complication occurs when activated CAR-T cells release large amounts of cytokines (signaling molecules) into the bloodstream.
Neurotoxicity: Some patients may experience neurological side effects, including confusion, seizures, and delirium.
Limited use: CAR-T Cell Therapy is currently approved for certain types of leukemia. Its application in solid tumors is limited due to issues such as tumor heterogeneity and difficulties in targeting solid tumor-specific antigens.
Tumor-Infiltrating Lymphocyte Therapy
Tumor-infiltrating lymphocytes (TIL) therapy is an immunotherapy approach used in the treatment of certain types of cancer, particularly solid tumors. TIL therapy involves extracting immune system T lymphocytes from the patient’s tumor and selecting and expanding cells that specifically target the cancer.

Examples of Tumor-Infiltrating Lymphocyte Therapy Include:
Melanoma treatment: TIL therapy has been extensively studied in advanced melanoma. Some clinical trials have shown promising results, leading to durable responses in a subset of patients.
Cervical cancer treatment: TIL therapy has also been investigated in cervical cancer. A small number of patients with advanced cervical cancer have responded to TIL therapy.
The advantages of treatment with tumor-infiltrating lymphocytes are:
Personal treatment: TIL treatment is personalized for each patient. The extracted lymphocytes are specific to the patient’s tumor and potentially increase the effectiveness of the treatment.
Natural infiltration: TILs are derived from the tumor itself, indicating the ability of these lymphocytes to infiltrate the tumor environment.
Permanent treatment: Some patients treated with TILs have experienced long-lasting responses, indicating the potential of this therapy to provide sustained anticancer effects.
The Disadvantages of treatment with tumor-infiltrating lymphocytes:
Limited use: TIL therapy has been more successful in certain types of cancer, such as melanoma, where TILs are more abundant. The efficacy of this treatment in other solid tumors may be more limited due to issues such as insufficient TIL infiltration.
Complexity and diversity: TIL treatment involves several steps, including extraction, proliferation, and re-infusion. Variability in the quality and quantity of TILs obtained from each patient can affect treatment outcomes.
Side effects related to the immune system: Like other immune-based therapies, TIL therapy can lead to immune-related side effects, including cytokine release syndrome and the development of autoimmune diseases.
Lack of specificity: While TILs may infiltrate the tumor, they do not exclusively target cancer cells. This lack of specificity can lead to damage to the healthy tissues as well.
Tumor heterogeneity: Solid tumors often show a heterogeneous structure, meaning that different regions of the tumor may have distinct characteristics. TILs obtained from one part of the tumor may not affect other parts of the tumor.
Cancer Vaccines
Cancer vaccines are a type of immunotherapy that aims to stimulate the patient’s immune system to identify and target cancer cells more effectively. Cancer vaccines are made of dead cancer cells, proteins or parts of cancer cells or cells of the immune system. By injecting into the patient’s body, these vaccines introduce tumor antigens to the immune system, and through this, the immune system cells recognize and remember these antigens.
Cancer vaccines can be divided into two main categories:
Preventive cancer vaccines: These vaccines are designed to prevent cancer in people who are at high risk of developing cancer due to factors such as family history or genetic predisposition. The most well-known example of this type of vaccine is the HPV (human papillomavirus) vaccine, which helps prevent certain types of HPV infections that can lead to cervical and other cancers.
Cancer therapeutic vaccines: The goal of these vaccines is to treat existing cancer by increasing the immune system’s ability to identify and attack cancer cells. Cancer
Therapeutic Vaccines can be customized to target specific antigens present in the patient’s cancer cells.
Benefits of cancer vaccines:
Targeted approach: Cancer vaccines can be designed to target specific antigens on cancer cells and reduce the risk of harming healthy cells.
Long-term immune response: A successful vaccine can lead to the development of memory immune cells, meaning the immune system can continue to target cancer cells even after initial treatment.
Reduction in side effects: Compared to traditional treatments such as chemotherapy, cancer vaccines may have fewer systemic side effects because they are designed to target only cancer cells.
Disadvantages of cancer vaccines:
Limited effectiveness: Cancer cells often develop mechanisms to evade the immune system, which can limit the effectiveness of vaccines.
Patient’s variability: Cancer vaccine success can vary among patients due to differences in immune response and specific characteristics of their cancer.
High costs: Developing and testing cancer vaccines is a time-consuming and expensive process.
Cytokine Therapy
Cytokine therapy is a type of immunotherapy that involves the use of special signaling molecules called cytokines to stimulate a person’s immune system response against cancer cells. Cytokines are proteins that regulate immune responses, cell growth, and inflammation. The two major types of cytokines used in cancer treatment are interleukins and interferons.
Interleukins are cytokines that help regulate immune responses. IL-2 and IL-12 are commonly used in cancer treatment.
- IL-2 stimulates the growth and activation of various immune cells, including T cells and natural killer (NK) cells. High-dose aldesleukin is used to treat advanced melanoma and renal cell carcinoma.
- IL-12 enhances the anti-tumor activity of the immune system by increasing the production of gamma interferon.
- Interferon therapy: Interferons are proteins that interfere with viral replication and modulate immune responses. Alpha interferon is used in cancer treatment to stimulate immune cells and inhibit the growth of cancer cells to treat certain types of leukemia, lymphoma, and melanoma.
Benefits of using cytokines
Immune Response Stimulation: Cytokines can increase the activity of immune cells and lead to a stronger immune response against cancer cells.
Long-term responses: In some cases, cytokine therapy can lead to durable responses and long-term recovery.
Vast usage: Cytokine therapy can potentially be used for many types of cancer because it targets the immune system’s response instead of directly attacking specific cancer cells. This versatility is especially valuable for cancers that may not respond well to traditional therapies.
Systemic effect: Cytokine therapy can target primary and metastatic tumors throughout the body. This systemic effect is especially beneficial when dealing with cancers that have spread to multiple sites.
Disadvantages of using cytokines
High Toxicity: The use of high-dose cytokine therapy can lead to severe side effects, including fever, chills, fatigue, muscle aches, low blood cell counts, and organ toxicity.
Capillary leak syndrome: High doses of cytokines, such as interleukin-2, can lead to a condition called capillary leak syndrome. This condition causes permeability of blood vessels and leads to leakage of fluid into tissues and organs, which leads to edema, low blood pressure and dysfunction of organs.
Blood Toxicity: Cytokine therapy can suppress bone marrow function and lead to a decrease in the production of blood cells, including red blood cells, white blood cells, and platelets.
Autoimmune reactions: The activation of the immune system caused by cytokines can sometimes lead to autoimmune reactions in which the immune system attacks normal tissues in addition to cancer cells.
Resistance: Some cancers may become resistant to cytokine therapy over time.
Duration of immunotherapy
The course of immunotherapy treatment in cancer can be influenced by several factors, including:
- Type of cancer
- Type of immunotherapy
- Individual patient response to treatment
- Stage of the cancer
Based on these factors, immunotherapy can include short-term to long-term treatment regimens.
Short-Term Treatment (Weeks to Months)
Some patients may receive a short course of immunotherapy, usually lasting a few weeks to a few months. This approach is common for certain types of cancer, especially when combined with other treatments such as chemotherapy or radiation therapy. Short-term immunotherapy may be used to rapidly boost the immune response, induce tumor shrinkage, or prepare the tumor microenvironment for other therapies.
Medium-term treatment (several months to a year)
Many patients undergo an intermediate-term immunotherapy regimen that typically lasts from a few months to a year. This period gives the immune system enough time to respond to the treatment, leading to shrinkage and potential stabilization of the tumor.
Long-term treatment
Some patients with advanced or metastatic cancers may continue to receive immunotherapy on an ongoing basis as a maintenance treatment. The goal of long-term treatment is to control the disease, prevent further tumor growth and maintain the patient’s quality of life.
Adjuvant therapy (after surgery or other treatments)
In some cases, immunotherapy is used as adjunctive therapy after surgery or other primary treatments to reduce the risk of cancer recurrence. Adjuvant therapy can be continued for a certain period to ensure that the remaining cancer cells are targeted. It is important to note that immunotherapy does not always follow a strict schedule, as each patient’s response to treatment is unique. Some patients may respond quickly to treatment, while others may need more time. In cases where treatment is effective, doctors may continue immunotherapy until there is evidence of disease progression.
Side effects related to the immune system
In immunotherapy, the immune system attacks healthy tissues in addition to cancer cells. Associated side effects include skin rash, diarrhea, colitis, hepatitis, thyroid dysfunction, and pneumonitis.
Advantages and Disadvantages of Immunotherapy
Immunotherapy, using the body’s immune system to target cancer cells, has revolutionized cancer treatment. However, like all medical interventions, immunotherapy can lead to side effects too. Specific side effects vary based on the type of immunotherapy, the individual patient’s response, and the type of cancer being treated. Here are some common side effects associated with immunotherapy:
Skin reactions: Skin-related side effects can include rashes, itching, and blisters. Some patients may develop certain skin conditions such as dermatitis or vitiligo due to the immune response.
Digestive problems: Diarrhea, nausea, vomiting and abdominal pain are possible side effects. Severe cases can lead to colitis.
Endocrine dysfunction: Some immunotherapies can affect the endocrine system, leading to hormonal imbalances and conditions such as thyroid dysfunction or adrenal insufficiency.
Pneumonitis: In some cases, as a result of immunotherapy, inflammation of the lungs (pneumonitis) occurs and symptoms such as shortness of breath, cough or chest pain develop.
Flu-like symptoms: Some patients may experience flu-like symptoms, including fever, chills, muscle aches, and headache. These symptoms are often related to the immune response. It is important to note that although the side effects of immunotherapy can be challenging, they are usually manageable with early diagnosis and appropriate intervention.
Conclusion
The potential of immunotherapy to be combined with other therapies has expanded the treatment options for patients in various types of cancer, including those with limited treatment alternatives. However, like any medical intervention, immunotherapy also faces challenges. Patient responses can vary widely, and safety-related side effects require careful monitoring and management. Continuous follow-up, modification of treatment protocols, proper patient selection and discovery of the complexities of immune system interactions with cancer cells are still the main goals of research.