Home » Microbiome and Probiotics » Engineered Microbiome-Friendly Phages
Microbiome: The Peaceful Coexistence of Microorganisms and Body Cells
The human microbiome, or the coexisting microorganisms of humans, refers to all the genes and populations of microbial cells and fungi that live within the host’s body. They play a beneficial role in certain natural bodily functions and are even essential in some cases. The microbiome has a significant role in maintaining human health, and any imbalance in it can lead to various diseases. The first scientific evidence that microorganisms are part of the human natural system emerged in the mid-1880s. During that decade, a pediatric specialist named Theodor Escherich observed a type of bacteria in the intestinal flora of healthy children and children suffering from diarrhea. This bacterium was later named Escherichia coli. In a healthy individual’s body, harmful and beneficial microbes coexist without causing any problems. Antibiotics are the primary treatment for bacterial infections that can lead to infectious diseases. However, excessive use of antibiotics can disrupt the functioning of beneficial bacteria present in the microbiome and disturb the balance between beneficial and harmful microbes in the body. As a result, the body becomes more susceptible to diseases.
Bacteriophages, or phages, offer a promising alternative to antibiotics. Creating phages engineered to be compatible with the microbiome provides a selective and non-harmful approach to selectively eliminating harmful bacteria from the body without causing damage to the microbiome. In this article, we review recent advancements in the development and application of microbiome-friendly engineered phages for targeted eliminate of harmful bacteria.
Contents
Microbiome-Friendly Phages
The human microbiome is a complex community of microorganisms that play a fundamental role in human health and disease. It consists of bacteria, viruses, fungi, and other microbes that reside in various parts of the body, such as the gut, skin, and mouth. Maintaining a balanced microbiome is crucial for human health, and any imbalance can lead to various diseases, including inflammatory bowel disease, allergies, and even cancer. Harmful bacteria present in the microbiome can cause infection and illness, and antibiotics are the primary treatment for such infections. However, antibiotics can disrupt the functioning of beneficial bacteria in the microbiome and lead to serious complications. Therefore, studying alternative approaches to antibiotics is essential.
Bacteriophages, which are viruses that specifically infect bacteria and eliminate them, have been known for over a century. Bacteriophages have selective behavior and can even target antibiotic-resistant bacteria. However, the use of phages can also impact the microbiome, highlighting the need for the development of microbiome-compatible engineered phages.
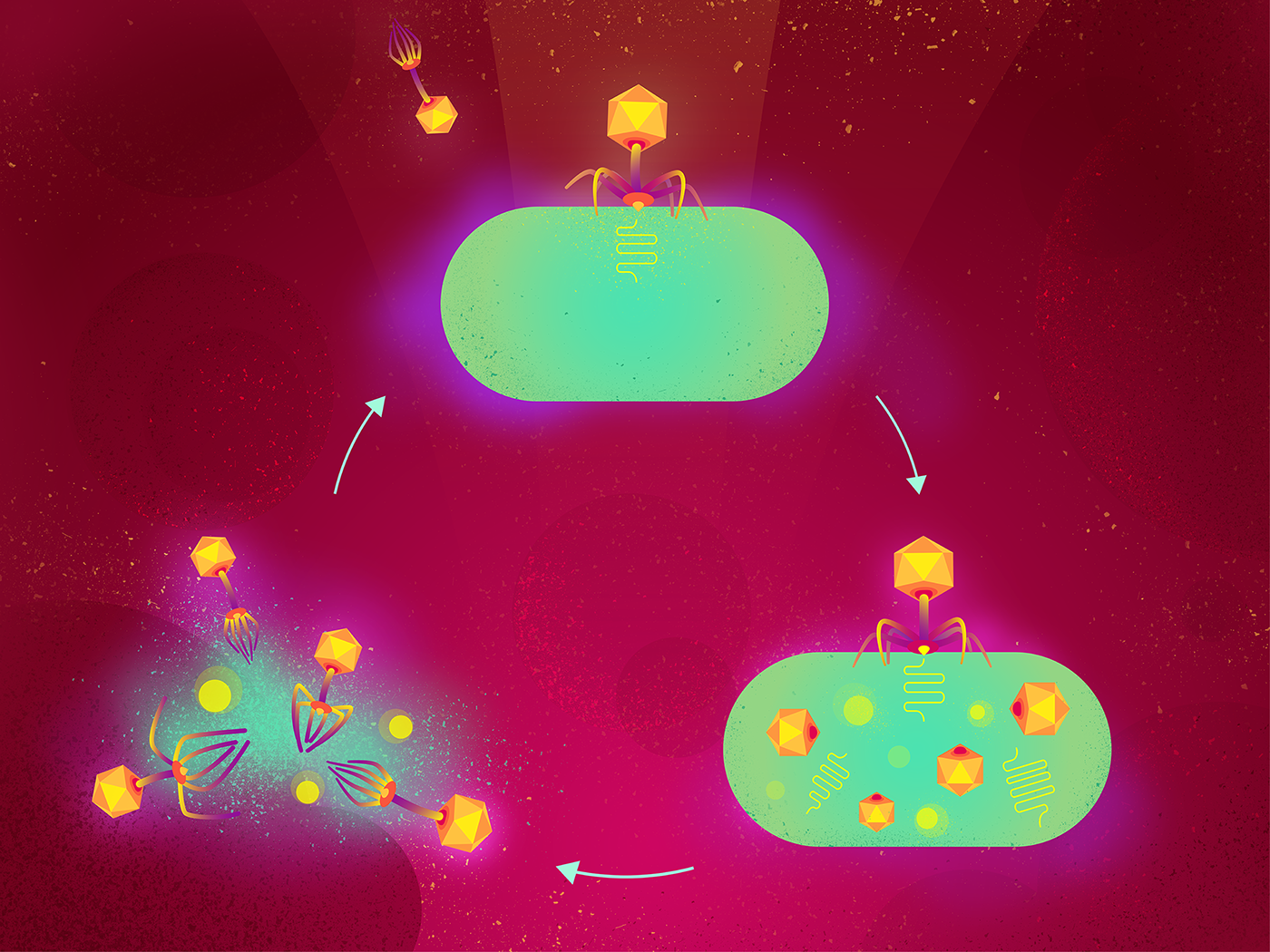
Applications of Engineered Phages
Engineered phages are designed to specifically target and eliminate specific harmful bacteria while leaving beneficial bacteria untouched. The development of such phages involves several stages, including identification of target bacteria, isolation of specific phages for the target bacteria, and modification of the phages for compatibility with the microbiome. This modification can be achieved through various techniques, including genome editing, chemical modification, and biotechnological engineering. Microbiome-friendly engineered phages have been utilized in various applications, including food safety, animal health, and human health. In the food industry, phages are used to prevent the growth of harmful bacteria in food products. In animal health, phages are employed to treat bacterial infections in livestock, leading to improved health and reduced antibiotic usage. In human health, phages have been used to treat bacterial infections, including those caused by antibiotic-resistant bacteria. Engineered phages have also been used in clinical trials for the treatment of various diseases, such as urinary tract infections, skin infections, and respiratory infections.
Engineered Phages and the Future Outlook
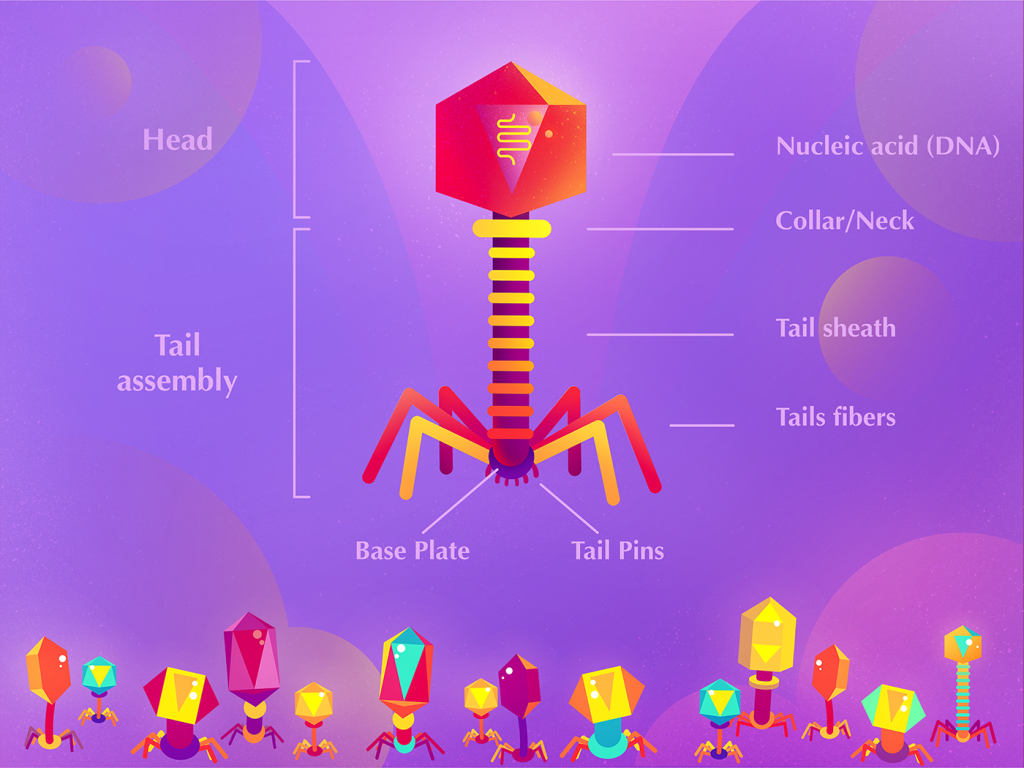
Researchers are currently utilizing therapeutic phages to treat Pseudomonas aeruginosa infections in cystic fibrosis patients. While pharmaceutical companies and major investors are not rushing to embrace phage therapy, the growing evidence of their effectiveness, particularly against drug-resistant bacteria, will change this situation.
Antibiotics can treat the skin condition of acne vulgaris, but they also eliminate beneficial C. Acne bacteria involved in skin homeostasis. Scientists have successfully designed phages using the CRISPR genome editing system to selectively remove strains of Cutibacterium acnes that produce the pro-inflammatory peptide CAMP1. The goal of this therapeutic approach is to preserve beneficial bacteria while eliminating harmful strains.
Despite the increasing excitement and inclination toward exploring new approaches in the field of microbiome and phage therapy, regulatory obstacles still remain. Currently, there are no guidelines or infrastructure in place for phage therapy products to be used in clinics. Nevertheless, the growing evidence of phage therapy’s effectiveness against multidrug-resistant bacteria confirms its potential, shifting the focus toward preserving beneficial microbiome strains and making phage therapy a promising area for research and investment.
 Researchers are currently utilizing therapeutic phages to treat Pseudomonas aeruginosa infections in cystic fibrosis patients. While pharmaceutical companies and major investors are not rushing to embrace phage therapy, the growing evidence of their effectiveness, particularly against drug-resistant bacteria, will change this situation.
Researchers are currently utilizing therapeutic phages to treat Pseudomonas aeruginosa infections in cystic fibrosis patients. While pharmaceutical companies and major investors are not rushing to embrace phage therapy, the growing evidence of their effectiveness, particularly against drug-resistant bacteria, will change this situation.
Antibiotics can treat the skin condition of acne vulgaris, but they also eliminate beneficial C. Acne bacteria involved in skin homeostasis. Scientists have successfully designed phages using the CRISPR genome editing system to selectively remove strains of Cutibacterium acnes that produce the pro-inflammatory peptide CAMP1. The goal of this therapeutic approach is to preserve beneficial bacteria while eliminating harmful strains.
Despite the increasing excitement and inclination toward exploring new approaches in the field of microbiome and phage therapy, regulatory obstacles still remain. Currently, there are no guidelines or infrastructure in place for phage therapy products to be used in clinics. Nevertheless, the growing evidence of phage therapy’s effectiveness against multidrug-resistant bacteria confirms its potential, shifting the focus toward preserving beneficial microbiome strains and making phage therapy a promising area for research and investment.